

A report published by the Health & Social Care Committee has illuminated the decision-making process behind vaccine authorisation, which was undertaken by the new Medicines Committee – a group formally established in September 2020.
The Medicines Committee is a public body which has been established by the Health & Social Care department. It did this through powers granted to it by the Human and Veterinary Medicines Law of 2008.
HSC note that it is “designed to bring together experts in their relevant fields to advise the Department on matters relating to medicines and the execution of the Medicines Law.
“The planned commencement of a COVID-19 vaccination programme necessitated the establishment of the statutory Medicines Committee so that formal advice could be provided on the potential designation of vaccines.”
The Committee has responsibility to advise on the execution of the Medicines Law, the exercise of power through the Law, and on issues relating to the regulation of medicines.
This includes ensuring the safety and efficacy of medicinal products and their subsequent distribution in the Bailiwick. The Committee must also investigate the adverse side effects of any medicine to fulfil its obligations as set out above.

Pictured: The functions of the Medicine Committee are set out in the report.
Whilst the Law has been active since 2009, the Medicines Committee only came into formal operation in September 2020, with its core initial task to review and provide recommendations to HSC on covid-19 vaccines.
Its membership aims to combine expertise across the spectrum of pharmacy, public health, veterinary and nursing locally. Together they evaluate the latest scientific research along with the necessary governance required for implementing new medicinal products.
Membership of the Committee currently includes, but is not limited to, the Chief Pharmacist, the Director of Public Health, the States Veterinarian, a senior nurse, and a senior civil servant.
Whilst advising on the designation of covid-19 vaccine products, the Committee followed an agreed process of authorisation underscored by the actions of its UK counterparts. This process was compulsory before Guernsey’s Health & Social Care Committee could consider any vaccine for human use.
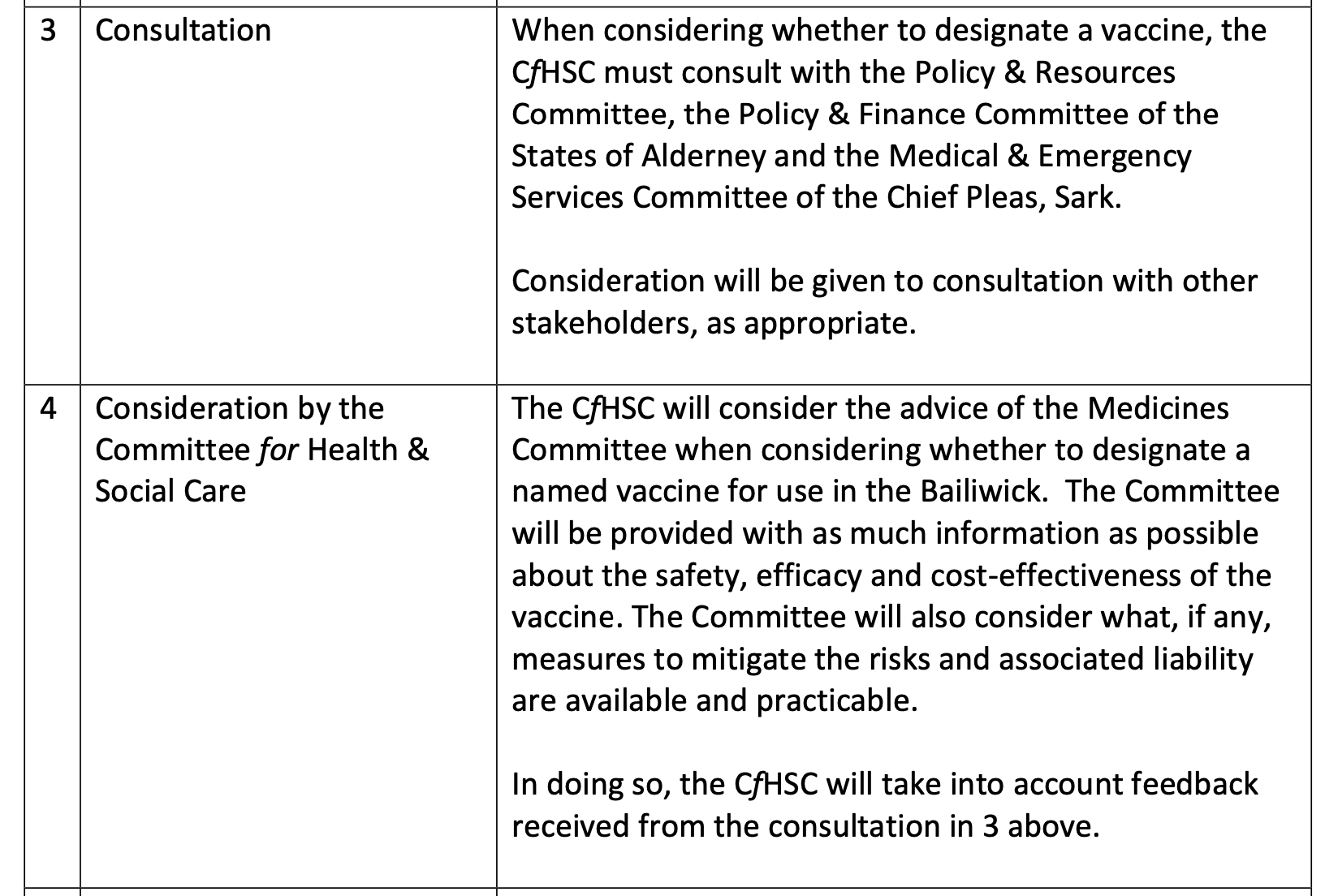
Pictured: A detailed breakdown of the vaccine designation process is set out in the report, showing interactions between local public health bodies, political representatives and their UK counterparts.
The Medicines Committee studied information published by the UK’s Medicines and Healthcare products Regulatory Agency as well as the Joint Committee on Vaccination and Immunisation. They concluded, based on the available evidence, “that the vaccines were safe, well-tolerated and had a high efficacy".
Three vaccine products were subsequently recommended for use by the Committee across December 2020 and January 2021: Pfizer/BioNTech, AstraZeneca, and Moderna vaccines.
They also stated after approving the medicines for public use that “it would keep under review any emerging evidence both locally and from other jurisdictions in respect of adverse reactions associated with the vaccine".
The Committee remains steadfast in its belief, based on weekly reviews, that “the expected benefits of the vaccines in preventing covid-19 and serious complications associated with covid-19 far outweigh any currently known side effects".
The Committee took specific action in the case of the AstraZeneca vaccine when it was discovered that there is a “more severe, and extremely rare, potential side effect” involving a blood clot with low blood platelets. It therefore decided to restrict the use of the vaccine for those under the age of 30.

Pictured: The Medicines Committee recommended that the AstraZeneca vaccine should not be given to those under 30 due to the possibility of a rare side effect, this was later implemented by the Committee for Health and Social Care.
HSC claim that “recent experiences have demonstrated the value of ensuring laws remain reflective of ever-evolving clinical practice and appropriately aligned to equivalent UK provisions.
“The Medicines Committee fully supports any steps to ensure that the legislative framework locally is responsive and flexible.”
The body continues to operate and should convene early this year to further define its operations.
This includes establishing its terms of reference, the frequency and schedule of its yearly meetings, the appointment of a lay member, and to review and learn from its activities from the previous year.
The annual report can be viewed HERE.
Comments
Comments on this story express the views of the commentator only, not Bailiwick Publishing. We are unable to guarantee the accuracy of any of those comments.